Nadrich Accident Injury Lawyers is actively representing those who have developed Pseudomonas aeruginosa bacterial infections after using EzriCare Artificial Tears.
According to the Food & Drug Administration (FDA), the eye drops may be contaminated with bacteria, and the Centers for Disease Control and Prevention (CDC) has identified 68 patients located in 16 states who have developed eye infections. Three people have died, eight have lost their vision and four people have had eyeballs removed. Global Pharma HealthCare Private Limited initiated a recall of the potentially contaminated eye drops on February 2, 2023.
Call us today or text us from this page for a free consultation if you or a loved one developed a Pseudomonas aeruginosa eye infection after using EzriCare Artificial Tears. You may be eligible for financial compensation in an EzriCare Artificial Tears eye drops lawsuit. Our legal team does not charge a fee until and unless we obtain compensation for you.
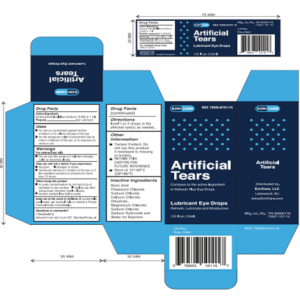
What EzriCare Eye Drops May Be Contaminated?
The eye drops which may be contaminated are:
- EzriCare Artificial Tears – product codes UPC 3 79503 10115 7, NDC 79503-0101-15
Can I File An EzriCare Lawsuit?
You may qualify to file an EzriCare eye infection lawsuit if you or someone you love:
- Used EzriCare Artificial Tears
- Subsequently suffered injuries such as vision loss or bacterial infection
However, there is a time limit for you to take legal action. This time limit is known as a statute of limitations. This time limit varies from state to state. A failure to file a lawsuit within this time limit may forever bar you from obtaining financial compensation for your Pseudomonas aeruginosa infection.
Don’t delay if you don’t want to miss out on holding Global Pharma responsible for your EzriCare Artificial Tears eye infection. Call us today for a free case evaluation.
What Is Pseudomonas Aeruginosa?
Pseudomonas aeruginosa is a bacteria which can cause infections in humans. It is a bacteria known for its resistance to many antibiotics as well as the serious illnesses it can cause.
The bacteria has been associated with pneumonia, sepsis and bacteremia, which is 18 to 61 percent fatal. Those who develop Pseudomonas aeruginosa eye infections may suffer from permanent vision loss or even death.
The bacteria can spread to other body parts or organs after it infects the eye. The bacteria has caused severe infections in those who have used eye drops manufactured by Global Pharma, according to lawsuits.
Is There An EzriCare Artificial Tears Recall?
Global Pharma has recalled EzriCare Artificial Tears due to the possibility of bacterial contamination, requesting that everyone who has these products, including retailers, wholesalers and customers, should stop using the product.
Global Pharma has also recalled Delsam Pharma Artificial Tears and Artificial Eye Drops.
Who May Be Held Liable In An EzriCare Eye Drops Lawsuit?
EzriCare Artificial Tears lubricating eye drops are made by Global Pharma, a company based out of India. The company makes numerous brands of healthcare products, including the EzriCare Artificial Tears brand, in India.
Global Pharma, obviously, may be held liable in an EzriCare recall lawsuit. However, that is not the only company who may be held liable in infection lawsuits.
The FDA, prior to the recall of EzriCare’s lubricant eye drops, placed Global Pharma on import alert, a “red list” indicating that the manufacturer has problems.
According to the FDA, Global Pharma provided an insufficient response to a request for records, and committed multiple violations of good manufacturing practices, including not performing appropriate microbial testing, distributing the eye drops in multiple-use bottles without including a proper preservative, and not having proper controls in place regarding packaging which is tamper-evident.
The fact that the FDA red-listed Global Pharma prior to the recall may be important – it means that retailers such as Walmart and Amazon may also be held liable in an EzriCare Artificial Tears lawsuit.
What did retailers like Amazon and Walmart do after learning about the issues with Global Pharma? Did they simply ignore them?
What States Have Been Affected By The Eye Drops?
As of March 21, 2023, bacterial infections have been reported in 16 states: Wisconsin, Washington, Utah, Texas, South Dakota, Pennsylvania, Nevada, New York, New Mexico, New Jersey, North Carolina, Illinois, Florida, Connecticut, Colorado and California.
What Are The Symptoms Of An EzriCare Artificial Tears Eye Infection?
If you have developed an eye infection after using EzriCare Artificial Tears, your early symptoms might include:
- Eye discomfort
- Clear, green or yellow fluids that leak from your eye
- The sensation of having a foreign body inside your eye
- Eyelid or eye redness
- Blurry vision
- Sensitivity to light
If the bacteria spreads from the eye, you may develop the following symptoms:
- Endophthalmitis, an infection in your eyeball which may lead to permanent blindness
- Keratitis, which is cornea inflammation
- Urinary tract infections
- Respiratory infections
- Hospitalization
- Sepsis
- Death
NBC News has reported that one patient has died and three have gone blind in an eye.
How Much Do EzriCare Lawyers Cost?
If you have suffered permanent vision loss, severe infection or other illness after using eye drops manufactured by Global Pharma, our lawyers will represent you without ever charging you a single penny out of your own pocket.
We will offer you a free, no obligation case evaluation and won’t charge you a fee until and unless we obtain financial compensation for you. The only fee we charge to handle your legal claim is a percentage of any compensation we obtain for you.
Call us today for a free case review.
Ezricare Artificial Tears Lawsuit Updates
June 10, 2024 Update
A lawsuit filed in New Jersey by a Massachusetts woman alleges the woman ended up with debilitating and permanent damage to her vision from Ezricare Artificial Tears which she purchased on Amazon. The lawsuit alleges the eye drops, which have been recalled, were contaminated with bacteria.
The lawsuit alleges the woman’s severe eye infection was the result of the manufacturer and retailer failing to properly test the eye drops for the presence of bacteria.
The lawsuit alleges that after the woman used the eye drops, she experienced burning, sharp pain, itching and redness, and started seeing flashes of light and floaters. According to the lawsuit, the woman ended up being diagnosed with blurred vision, posterior vitreous detachment, ocular headaches, double vision and dry eye syndrome.
May 21, 2024 Update
EzriCare has failed to convince a federal judge in Kentucky to dismiss a lawsuit which claims the company failed to warn its customers regarding bacterial contamination of its artificial tears. The judge considered the complaint to be sufficiently clear in order for the company to understand the role it had in any alleged offenses.
The lawsuit claims a woman developed sepsis after developing an infection from the eyedrops, leading to worsening eyesight and numerous surgeries. The lawsuit claims the company knew its products were contaminated with bacteria but failed to warn customers.
August 3, 2023 Update
A lawsuit filed in Pennsylvania on August 3, 2023 alleges that a woman needed to have her eye removed and replaced with a plastic eye implant after she used Ezricare Artificial Tears. The lawsuit alleges the eyedrops caused her to develop a bacterial infection which eventually necessitated the removal of her eye.
July 17, 2023 Update
On July 17, 2023, Bloomberg News reported that the recalled artificial tears were never tested by the FDA, and that the overseas facility where the artificial tears were manufactured was never inspected by the FDA. The report highlights how some products sold in the United States over-the-counter don’t require their makers to provide any evidence that establishes they are effective or safe. The report also highlights how product makers aren’t always made to have their packing plants or production plants inspected by the FDA before their products are distributed throughout America. The report claims that all companies need to do is tell the agency that it uses approved ingredients and follows proper manufacturing practices. The FDA apparently doesn’t check if manufacturer claims are accurate until a problem occurs. In effect, this is an honor system.
June 1, 2023 Update
A lawsuit filed in the Central District of California, Southern Division claims a California man developed a severe infection as well as permanent damage to his vision after using EzriCare Artificial Tears.
April 1, 2023 Update
A medical journal case report describes a corneal ulcer associated with EzriCare Artificial Tears.
Another medical journal case report describes keratitis associated with EzriCare Artificial Tears.
March 2, 2023 Update
The FDA released an inspection report regarding an inspection of a Global Pharma Healthcare plant located in India. The inspectors found numerous issues at the plant, including an unvalidated sterilization process, deficiencies in the aseptic cleanroom processing areas of the plant, test methods which haven’t been adequately verified, inappropriately designed equipment, a lack of written procedures for maintenance and cleaning of equipment, and insufficient quality control measures.
March 1, 2023 Update
Global Pharma Healthcare recalls EzriCare Artificial Tears due to potential Pseudomonas aeruginosa contamination.
A class action lawsuit is filed over the bacterial contamination against EzriCare LLC, Delsam Pharma LLC, EzriRx LLC, Aru Pharma Inc., and Global Pharma Healthcare Private Ltd.
A separate class action lawsuit is filed over the contamination against EzriCare LLC and Delsam Pharma LLC.
An individual lawsuit is filed over the bacterial contamination against EzriCare LLC, Wal-Mart Stores East LP, Walmart, Inc., Aru Pharma Inc. and EzriRx LLC. Global Pharma had been “red-listed” by the FDA prior to the recall of the eyedrops, and this means more future lawsuits may name retailers such as Walmart as defendants.
The AP reports that an outbreak of eye infections in a Connecticut nursing home helped officials discover the possible contamination of EzriCare Artificial Tears.
How Do I File An EzriCare Artificial Tears Lawsuit?
At Nadrich Accident Injury Lawyers, we’re aware of how difficult things must be for you and your loved ones right now. We will make the process of filing an EzriCare eye drops lawsuit as free of stress as we can by taking care of every step of the lawsuit process for you, and without charging you any out-of-pocket fee.
Our lawyers have been handling dangerous drug cases like this for over 30 years and have recovered over $750,000,000 for our clients, including $14,250,000 for a child who developed a life-threatening condition after taking Children’s Motrin. When you work with our experienced EzriCare attorneys, you will have to do very little to file a lawsuit.
Our law firm can:
- Verify that you are eligible to file a lawsuit by giving you a free consultation
- Collect evidence like testimony and medical records to put together an extremely strong case
- File a lawsuit for you, making sure any applicable deadlines are met
- Negotiate a settlement with any defendants
- If need be, represent you in court where you will receive a verdict
Call us now for a free case evaluation. Allow us to recover the compensation you deserve. You will only owe us a fee if we win your case or obtain a settlement for you.

