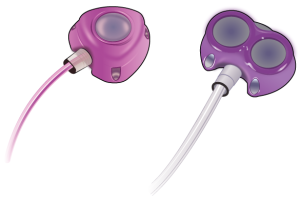
The Bard PowerPort is an implantable port device made and sold by Bard Access Systems, Inc. It’s designed to let medical professionals easily inject fluids and medications into a patient’s bloodstream. If these devices fracture, become dislodged, or cause an infection in a patient’s body, though, they can cause serious harm.
Enough Bard PowerPort lawsuits have been already filed that a multidistrict litigation has been created to streamline the process of handling the lawsuits for the court system.
If you or someone you loved was injured or suffered health issues as a result of using a Bard PowerPort, you may be eligible for financial compensation. The PowerPort injury lawyers at Nadrich Accident Injury Lawyers are ready to fight for you. We work quickly and thoroughly, handling every step of the legal process so that you can focus on healing. We have been representing victims of defective medical devices for over 30 years. Contact us today for a free consultation to discuss your Bard PowerPort case.
What Is The Bard PowerPort Port Catheter Device?
The Bard PowerPort is a device that medical professionals implant under the skin of a patient’s arm or chest to easily access their veins. This implanted port device, also known as a port-a-cath, allows easy access to a patient’s vascular system and allows medical professionals to administer regular intravenous injections and therapies without sticking patients’ veins with needles.
Bard claims that its implanted port device can allegedly withstand higher injection pressures than a normal port, which allows for rapid fluid injection.
A Bard PowerPort port catheter device consists of:
- The port: This device has one or two small basins sealed with silicone that allow for multiple needle punctures. It gets implanted in the patient’s arm or chest, providing easy access for injections.
- The catheter: This is a small, soft tube that connects to the port. It is placed inside one of the large central veins that transports blood to the patient’s heart.
To administer medication or take blood samples using the PowerPort, a medical professional must use a special needle, called the PowerLoc needle, to stick the patient’s port and easily access their bloodstream.
It has been reported that the Bard PowerPort is implanted over 300,000 times per year in the United States, and that 59.05 percent of patients who have a port-a-catheter like a Bard PowerPort implanted in them see complications within five years. There are estimated to be 100,000 Bard PowerPort cases out there right now.
It has been reported that about 45 percent of the cases involve infection, about 35 percent of the cases involve breakage and about 30 percent of the cases involve blood clots.
Problems With Bard PowerPort Devices
Bard PowerPorts are designed to facilitate easier fluid and medication injections, but they may be subject to issues that cause them to fail.
Some injuries have occurred when metal needles have contacted plastic in the catheter, leading to small scratches and holes being created in the catheter. This lets bacteria collect on the catheter surface, possibly leading to severe or even deadly sepsis or infections.
The device also features plastic with barium sulfate mixed into it. While the barium sulfate is designed to make the device visible on imaging, lawsuits allege too much barium sulfate was used, leading to decreased structural integrity, device degradation and the possibility of the device fracturing and migrating within the body.
The three most common problems with PowerPorts are:
- Catheter fracture
- Catheter infection
- Catheter migration
Catheter Fracture
A catheter fracture is when a portion of a catheter breaks in a patient’s body. In some cases, the catheter may break down further within the bloodstream, causing silicone and polyurethane fragments to travel throughout the patient’s circulatory system.
Fractured catheters are often caused by flex fatigue, which is muscle flexing or movement around the site of the PowerPort.
Catheter Infection
Infections caused by PowerPort catheters can lead to serious and even life-threatening problems.
Common catheter infection injuries include:
- Bloodstream infections
- Tissue necrosis around the PowerPort
- Soreness or swelling where the PowerPort is placed
Catheter Migration
Migration is when a catheter dislodges itself from the port and moves to other organs in the patient’s circulatory system. Catheter migration may result in neck, shoulder, and ear pain, as well as various potentially life-threatening symptoms. Surgery may be required if the catheter migrates or the PowerPort device becomes dislodged.
Injuries Caused by Bard PowerPorts
Bard PowerPort devices may break apart or move around within the body, which could lead to serious injuries and health problems.
Common injuries associated with Bard PowerPorts include:
- Blood clots
- Severe infections
- Sepsis or septic shock
- Hematomas (pools of clotted blood that form in an organ or tissue)
- Cardiac punctures
- Deep vein thrombosis (DVT)
- Severe or chronic pain
- Necrosis
- Pulmonary embolism
- Patient death
What To Do After A Bard PowerPort Device Complication
If you’re experiencing pain or symptoms that you believe may be caused by your Bard PowerPort device, seek medical care immediately. Speak with your doctor to address the problem as soon as possible, and be sure to follow their orders to mitigate future complications.
If your injuries are the result of a Bard PowerPort, seek legal representation by contacting Nadrich Accident Injury Lawyers. Our Bard PowerPort lawyers understand what you’re going through and can help you navigate the legal process.
When you bring a Bard PowerPort claim to the team at Nadrich Accident Injury Lawyers, we can:
- Review your situation to determine if you qualify to file a lawsuit
- Gather evidence to build a solid case on your behalf
- File your lawsuit
- Negotiate to settle your case favorably
- Take your case to court if necessary
Is There A Time Limit To File A Bard PowerPort Lawsuit?
Each state’s statute of limitations determines how much time you have to file a lawsuit after suffering an injury associated with the Bard PowerPort. So, be sure to contact a trusted attorney as soon as possible.
Who Can File A Bard PowerPort Lawsuit?
You may be able to seek compensation by filing a Bard PowerPort lawsuit if you or a loved one suffered an injury or health problem associated with catheter failure or device dislodgement after using a Bard PowerPort.
You might have a Bard PowerPort case if you had the device implanted after 2007 and the device fractured or migrated causing:
- Stroke
- Heart attack
- Pulmonary embolism
- Hemorrhage
- Blood clot
- Arrhythmia
- Necrosis (tissue death)
- Bloodstream infection (sepsis)
- Vessel/organ perforation
Bard PowerPort Updates
March 5, 2025 Update
108 cases were added to the Bard PowerPort MDL in February, bringing the total number of cases to 1,112.
February 28, 2025 Update
The judge presiding over the Bard PowerPort MDL wants attorneys involved in the litigation to, next month, submit a joint proposal. The proposal will detail plans regarding getting initial bellwether trials ready to go before courts. The trials will help to determine how future juries might respond to allegations which will be repeatedly made during the litigation.
A case management conference will be held on March 20. The parties have been ordered to end up filing a joint memorandum two days or more prior to the meeting. The memorandum should detail a proposed schedule regarding bellwether-specific discovery, motions to exclude testimony from expert witnesses and motions regarding summary judgment.
December 2, 2024 Update
In November, a California man's estate executor filed a lawsuit alleging that the man's Bard PowerPort caused him to experience complications such as thrombosis, infection and catheter fracture.
While the man died, the lawsuit isn't a wrongful death lawsuit. Rather, the lawsuit is seeking compensation from the pain and suffering endured by the man, as well as the medical bills he accrued, prior to his death.
In other news, 93 cases were added to the PowerPort MDL in November, bringing the total number of pending cases to 813.
October 3, 2024 Update
September saw 94 new cases added to the Bard PowerPort MDL, which now involves 527 pending cases.
September 10, 2024 Update
The federal Bard PowerPort MDL is now more than one year old. Hundreds of claims now present similar accusations that the devices are defective by design and dangerous and that Bard failed to adequately disclose defects.
A docket entry which was issued on September 3 indicates at least 427 lawsuits have been filed in federal court against Bard. The lawsuits allege that the materials that the PowerPort devices are made from cause port catheters to degrade as well as develop pits, cracks and fissures.
Not only does this increase the risk of device breakage or fracture, but many lawsuits allege this promotes bacteria colonization, leading to:
- Sepsis
- Septic shock
- Staphylococcal infections
- Klebsiella
- Fungal infections
- Escherichia coli
- Endocarditis
- Candida
- Catheter-related bloodstream infections
The lawsuits alleges that air entrapped in catheters and barium sulfate deterioration can lead to pits, cracks and fissures where bacteria can thrive, causing bloodstream infections and blood clots. The lawsuits allege that the blood clots that the cracks and fractures in the catheters can cause can damage the walls of blood vessels, allowing bacteria additional ways to enter one's bloodstream.
A lawsuit filed in November 2023 alleges that a woman died from sepsis due to a defect in a PowerPort reservoir.
The lawsuits allege that Bard could have used safer designs including surface modifying additives, or SMA coatings. A 2009 study found that catheters with SMA coatings demonstrated significant reductions in bacterial proliferation and adhesion compared to uncoated catheters such as the catheters found in Bard PowerPort devices.
Lawsuits are seeking compensation for numerous side effects caused by infections, such as:
Antibiotic resistance: Infections can make the use of antibiotics possibly leading to antibiotic resistance which can lead to future infections being more difficult to treat.
Catheter malfunction: Infections may lead to malfunctions or blockages of catheters, necessitating emergency surgeries to replace or remove devices.
Thrombosis: Infections may lead to blood clot formation or thrombosis, obstructing blood flow and possibly causing pulmonary embolism in the event a clot travels into the lungs.
Port catheter infection symptoms include:
- Red streaks which extend from the site of the port
- Unexplained weight loss
- General malaise or fatigue
- Chills or fever
- Increased discomfort or pain around the site of the port
- Pus or drainage coming from the site of the port
- Heat or warmth around the site of the port
- Tenderness, swelling or redness around the site of the port
Call us today for a free consultation if you have suffered an infection due to a Bard PowerPort device.
In other news, a Vacaville, California woman has filed a lawsuit alleging that a Bard PowerPort's catheter was defective and caused her to develop a pericardial effusion and infection.
September 5, 2024 Update
The PowerPort MDL saw almost 100 cases added to it in August. This was the most cases added to the MDL in a single month since the beginning of the MDL. There are now 427 cases pending in the MDL.
August 26, 2024 Update
A recent filing in court shows how the scope and size of the Bard PowerPort litigation is expanding. At least 416 lawsuits involving the devices are now pending in federal court, and there are over 50 lawsuits in state courts.
The lawsuits allege that users experienced serious and life-threatening complications due to port catheters fracturing or small cracks developing in the devices, leading to bacteria forming and devastating infections developing.
August 12, 2024 Update
A case has been filed in the PowerPort MDL via a Short Form Complaint. The complaint claims a man received a BardPort M.R.I Implantable Port implantation in 2018. The man alleges this defective port device led to him developing an infection as well as vein thrombosis, making his case one with a high potential value.
July 31, 2024 Update
Since July 9th's monthly status conference, the litigation has seen answers to complaints filed in the MDL as well as an order which has pushed August's status conference back to the date of August 16th.
July 9, 2024 Update
Attorneys involved in the Bard PowerPort MDL met with the judge presiding over the MDL today in order to talk about plans regarding conducting discovery which is case-specific with regards to around two dozen bellwether trials which the parties selected last week. The bellwether claims are part of over 322 cases which are pending in the litigation.
The lawsuits involve similar claims that Bard port catheter devices had defective designs which led to complications such as fractures and severe infections. The lawsuits claim that Bard knew or should have known that the material that the devices' catheters are made of is likely to degrade, allowing the breaking off of small pieces or the development of bacteria.
An order will be coming soon which outlines case management as well as the initial bellwether trials' scheduling. The defendants and plaintiffs will then again meet on August 16 in court for another status conference.
July 2, 2024 Update
67 new cases were added to the Bard PowerPort MDL in June, bringing the total number of cases to 299.
We expected at the beginning that this litigation would be larger. However, the litigation being smaller than we expected is probably a good thing for plaintiffs regarding the willingness Bard will have to settle cases without attempting to delay litigation for a decade. Bard is just now reaching the finish line regarding settling its hernia mesh lawsuits and it's been very difficult to get Bard to reach settlements there.
June 5, 2024 Update
An Oklahoma woman has seen her lawsuit join the Bard PowerPort MDL. This planitiff had a PowerPort device implanted in 2017. Her lawsuit alleges that the defective device caused her to develop thrombosis and additional health complications. The lawsuit was directly filed in the MDL via the short form complaint.
June 3, 2024 Update
Steady growth is now being seen in the Bard PowerPort MDL. May saw 45 new cases added to the multidistrict litigation, bringing the total to 232 cases which are pending in the litigation.
May 10, 2024 Update
A status report was filed on May 7 by both parties in the Bard PowerPort MDL. The report indicates that at least 223 claims are currently pending in the litigation, and that 38 additional claims have been filed in New Jersey. Defendants are anticipating that they will file a motion seeking to transfer 29 of the New Jersey claims to federal court. As a result, in excess of 250 claims might be eligible for selection for bellwether trials.
The bellwether process will see 24 lawsuits go through discovery and undergo preparation for early trials.
Lawsuits in the MDL allege that Bard knew or should have been aware that the material that the catheters in the devices are made of can degrade over time, letting bacteria develop or leading to small pieces breaking off, increasing the risk of complications such as blood clots and infections.
May 6, 2024 Update
A new study found that venous port catheters made of polyurethane such as the Bard PowerPort have significantly shorter lifespans compared to catheters composed of silicone.
The study found that venous ports with polyurethane catheters were 1.7 times more likely to experience early failure than ports with silicone catheters.
The study comes as the number of Bard PowerPort lawsuits continues to grow. The lawsuits alleges that C.R. Bard knew or should have known that the polyurethane catheters in the Bard PowerPort is prone to degrading over time, which allows small pieces to come off or allows bacteria to develop, leading to pulmonary embolism or infection, as well as failure of port catheters and the necessity of revision surgery.
In other news, 50 cases were added to the Bard PowerPort MDL in April, bringing the total number of pending cases to 187.
April 21, 2024 Update
The process for bellwether trials in the Bard PowerPort MDL is proceeding on time.
Bard has argued that not enough lawsuits have been filed in order to select representative claims for bellwether trials. Bard argued that discovery proposed would be disproportionate in the event the MDL didn't grow as predicted by plaintiffs, and that the bellwether trial process would be based upon an insufficient number of cases in the MDL if the MDL didn't grow as predicted by plaintiffs.
The judge overseeing the MDL reviewed the joint report produced by the plaintiffs and defendants, reviewed IVC filter MDL statistics (and MDL similar to this one which the judge considered a relevant example), and heard extended comments from both parties. The judge then concluded that current expectations regarding discovery shouldn't be changed, and that the process regarding bellwether trial should continue to proceed as previously scheduled.
April 16, 2024 Update
A lawsuit has been filed by a Texas woman alleging she suffered atrial fibrillation after receiving a Bard PowerPort implant.
This injury is not as commonly reported regarding this device as are other injuries such as catheter fractures, thromboses and infections.
March 17, 2024 Update
The judge overseeing the Bard PowerPort MDL indicated the bellwether process will not be delayed, despite a manufacturer request. The manufacturer tried to present the argument that not enough lawsuits were filed to select bellwether trials.
Bard currently faces around 115 lawsuits alleging that users ended up developing blood clots, infections and additional complications after Bard PowerPorts were implanted. It is believed that it's likely that over 2,000 lawsuits will eventually be filed in the MDL.
March 9, 2024 Update
On February 27, a joint memorandum was filed indicating that 113 claims are pending in the Bard Powerport MDL. 45 of the claims were directly filed in the MDL court and 66 of the claims got transferred into the MDL from courts around the country.
The memo notes that:
- 44 of the claims involve infections
- 19 of the claims involve blood clots or thrombosis
- 27 lawsuits involve port catheter migration or fractures
- 9 claims involve port fractures and don't involve migration
February 14, 2024 Update
A judges' panel determined that the Bard Powerport MDL will include claims that allege that infections were caused by defects in the device's port reservoir. The MDL had been previously restricted to only claims involving catheter defects.
January 24, 2024 Update
Those who wish to see their lawsuits be considered to be included in a pool of bellwether trials have around two months left to file a Bard Powerport lawsuit. Bellwether trials in an MDL are the first trials, the trials which test evidence and allegations presented throughout the entire litigation, and can establish precedents for the amounts of money awarded in future trials.
December 11, 2023 Update
A judge has authorized direct filing of new lawsuits in the Bard Powerport MDL, which is being handled in Arizona. Plaintiffs can now file lawsuits directly in the MDL instead of filing them in district courts then waiting for their lawsuits to be moved into the MDL.
How Much Will It Cost To File A Bard PowerPort Lawsuit?
When you choose Nadrich Accident Injury Lawyers to handle your Bard PowerPort case, you won’t pay any upfront costs or hourly fees. There’s no risk associated with filing a lawsuit because our team will only get paid if you are rewarded financial compensation in your case.
Hire A Bard PowerPort Lawyer
If you’ve suffered an injury caused by a Bard PowerPort device, you shouldn’t have to face this challenge alone. The Bard injury attorneys at Nadrich Accident Injury Lawyers are prepared to represent you and fight for the compensation you deserve. We are filing Bard PowerPort lawsuits on behalf of those injured by the devices. We have been recovering compensation for those injured by the negligence of a medical device manufacturer since 1990. We have recovered over $750,000,000 for our clients in those 30+ years.
Contact a Bard PowerPort injury lawyer at Nadrich Accident Injury Lawyers today for a free consultation — give us a call, text us from this page, or fill out our contact form. We’ll get back to you quickly to advise you of your legal options.