Nadrich Accident Injury Lawyers is actively retaining those who have been seriously injured by defective JET 7 catheters, as well as the families of those who have died because of a defective JET 7 catheter.
Penumbra has issued an urgent recall of all configurations of their Penumbra Jet 7 Reperfusion Catheter with Xtra Flex Technology (Jet 7 Xtra Flex) because of the risk of unexpected serious injury or death when the product is used to remove clots from stroke patients.
Our representation is free unless and until we obtain financial compensation for you. Call us now at 800-718-4658 for a free consultation. You may be eligible for financial compensation if your loved one died due to a defective JET 7 catheter. You can also fill out the “Do I Have A Case” form on the right, email us at info@personalinjurylawcal.com or engage with a live chat specialist.
Defective Catheters
The defective catheter devices include:
- The JET 7 Xtra Flex catheter, originally cleared under K190010 on June 16, 2019.
- The JET 7MAX configuration (which includes the JET 7 Xtra Flex catheter and MAX Delivery Device) cleared under K191946 on February 27, 2020.
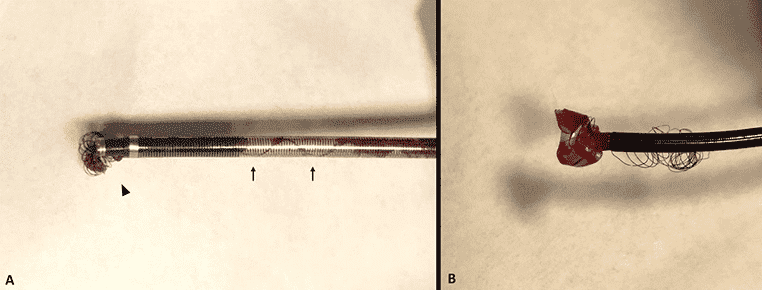
The FDA said they have received over 200 reports about the JET 7 catheter, including reports about deaths, malfunctions and serious injuries. 14 unique patient deaths are described by the reports, and other reports describe serious injuries like hemorrhage, cerebral infarction and vessel damage. Device malfunctions reported include expansion, ballooning, breakage, rupture, complete separation, and exposure of internal support coils.
The FDA said that manufacturer testing found the catheter can’t withstand the same burst pressures to failure as the manufacturer’s other large bore aspiration catheters which are used to remove clots from stroke patients.
Penumbra JET 7 Catheter Lawsuit Causes Of Action
We may be able to obtain financial compensation for you based on numerous causes of action in a JET 7 catheter lawsuit. The primary causes of action will be wrongful death, as well as strict liability causes based on failure to warn, design defect and/or manufacturing defect.
Wrongful Death
We may be able to obtain financial compensation for the wrongful death of your loved one as the result of a defective JET 7 catheter.
Failure To Warn
To establish a failure to warn claim in California against a product manufacturer, one must prove:
- The defendant made the product;
- The product had risks/side effects which the manufacturer knew or should have known about;
- The risks/side effects posed a substantial danger when the product is used as intended or misused in a reasonably foreseeable manner;
- Ordinary consumers won’t recognize the risks/side effects
- The defendant failed to adequately warn of the risks/side effects
- The plaintiff was harmed
- The lack of warning substantially caused the plaintiff’s harm
We believe Penumbra knew or should have known their JET 7 catheters posed an injury and death risk to patients. We believe they either knew the products couldn’t withstand the burst pressures their other similar products can, or should have known but didn’t because they inadequately tested their products. We believe Penumbra failed to warn the public about risks they knew or should have known about.
Design Defect
Products with a design defect are unreasonably dangerous when manufactured correctly and used as intended.
One must prove the following to establish a design defect claim in California against a manufacturer:
- The defendant manufactured the product;
- The plaintiff was harmed;
- The product’s design substantially caused the harm.
If these three things can be proven, juries are then instructed to decide whether the product’s benefits outweigh its risks, considering:
- The gravity of potential harm;
- The likelihood of harm;
- The feasibility of an alternative, safer design;
- The cost of an alternative design;
- The disadvantages of an alternative design;
- Other relevant factors.
We believe that the problems reported with the JET 7 catheters may be due to a design defect.
Manufacturing Defect
Products with a manufacturing defect are unreasonably dangerous because something unintended went wrong during the process of manufacturing the product. These products differ from the manufacturer’s design or specifications, or from other typical units in the same line of product.
One must prove the following to establish a manufacturing defect claim in California against a manufacturer:
- The defendant manufactured the product;
- The product contained a manufacturing defect when it left their hands;
- The plaintiff was hurt;
- The defect substantially caused the plaintiff to be hurt.
We believe that the problems reported with the JET 7 catheters may be due to a manufacturing defect.
Penumbra JET 7 Catheter Lawyers
We are defective medical device specialists and the defective medical device lawsuit experts. We have been actively retaining defective medical device victims since 1990. We have retained over $750,000,000 on behalf of clients in that time.
We only charge a percentage of your recovery as our fee. We only charge this fee if we obtain a recovery for you. We will not charge you a single penny if we do not obtain financial compensation for you.
Call us today at 800-718-4658 for a free consultation if your loved one died because of a defective Penumbra JET 7 catheter. You may be eligible for financial compensation.

